Today we are going to discuss this piece of special equipment called a rectification column. Ever thought of how one chemical is distinguished from the other? Well, that’s what a rectification column is for! It is integral to a process known as distillation.
Say you’ve got a pool of different types of chemicals you need to break apart. This liquid could be anything — say, oil, or alcohol. A rectification column helps with this by using heat to separate the chemicals according to how easily they boil. The chemicals are heated, and as they vaporize, they move up the column. Seperation can be made better or worse depending on the height of thr column.
Rectification columns are used for the efficient separation of chemicals. They contain trays or packing which provide a surface over which the air and vapor must pass in order to make contact with the liquid. This contributes to the resolution of the separation. It works just the way a reactor does, by regulating the temperature and pressure inside. Through altering these parameters, other chemicals can be better dissolved.

Components of a Rectification Column A rectification column consists of several major parts: the reboiler, the condenser, trays or packing, and a reflux drum. At the bottom of the column a reboiler serves to heat the mixture, and the top of the column a condenser cools the vapor. The trays or the packing serve to enhance the separation by introducing additional vapor and liquid space. The reflux drum decides how much of the liquid goes back into the column for further separation.
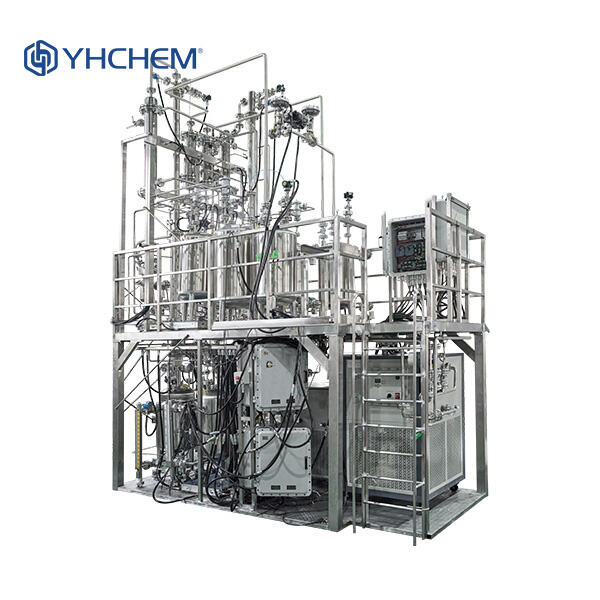
Temperature and pressure management is crucial in a rectification column. This is to ensure that the chemicals separate correctly. We can heat the chemicals up to their boiling point or cool them down to their boiling point through temperature control in the reboiler and condenser. The correct pressure within the column is also another important aspect for this technique.

If we optimise the design and operation of the rectification column, we can obtain very pure products. That is, the chemicals separated would exist as pure as they can be, minimally tainted by other chemicals. Optimization really involves changing things like temperature, pressure and reflux ratio to try to get the best separation. In the right configuration, a rectification column can generate top-quality products for several industries.
As a company has been listed successfully, We have stable financial stability sustainable development capability. We will remain committed principles that are based on market demand drive constant product innovation and technological advancement generate more opportunities and value rectification column, investors, and employees.
We provide range products including glassware temperature control, well reactions and rectification column equipment. Our product line specifically designed meet the needs of various industries. We offer customers all-in-one service that includes multiple service centers, supplying prompt technical assistance, after-sales services products.
Our products have assisted thousands well-known businesses more than 100 countries well as regions throughout rectification column, gaining the trust of many recognitions. We're committed to improving our products and services according to the feedback of our customers.
We have most rectification column technological innovation and R and D capabilities across the globe, constantly developing international technologies the future and continually conducting independent innovation technological upgrades. Through collaborations with well-known research institutes like Shanghai Chemical Industry Research Institute East China University Science and Technology We have established joint laboratories, dedicated to providing clients with the best competitive products solutions.